Abstract
Background: PLK1 is a serine/threonine kinase master regulator of the cell cycle, overexpressed in AML blasts. Onvansertib (ONV) is an oral, highly selective next-generation PLK1 inhibitor that demonstrated anti-tumor activity in AML preclinical models. The safety and efficacy of ONV in combination with decitabine (DAC) in patients with R/R AML is under study in an ongoing phase 1b/2 clinical trial (NCT03303339). Here we report results of correlative studies to identify molecular predictors of response to the ONV-DAC combination.
Methods: R/R AML patients were treated for 5 days with ONV (orally) and DAC (20 mg/m 2 IV qd x 5d) in 21- or 28-day cycles. Patients who completed at least 1 cycle of treatment were considered evaluable for efficacy. At baseline, bone marrow (BM) samples were collected for targeted next-generation sequencing (Myeloid VariantPlex, Archer) and blood samples for RNA-sequencing.
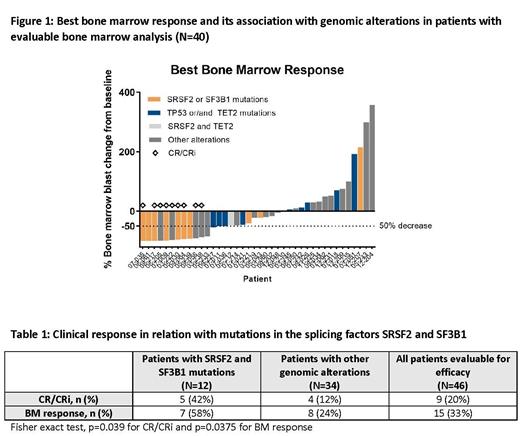
Results: As of July 7, 2021, 23 patients were treated with ONV (12 - 90 mg/m 2) + DAC in Phase 1b and 32 patients with ONV 60 mg/m 2 + DAC in Phase 2. Nine (20%) of the 46 patients that were evaluable for efficacy achieved a complete remission with or without complete hematologic recovery (CR/CRi) as their best response (7 CR and 2 CRi). Another 6 patients had ≥50% BM blast decrease from baseline at one point on trial therapy. Those 6 patients plus the 9 patients who achieved CR/CRi are referred to as BM responders (n=15). RNA-seq was performed on blood collected from 32 patients, including 12 BM responders. Differential gene expression, gene-set, and machine learning analyses of BM responders versus non-responders identified a gene expression signature predictive of response to the ONV-DAC combination. No association between the ONV-DAC signature and DAC response was observed after applying the ONV-DAC signature to gene expression profiles from patients treated with DAC as a single agent in a multicenter Phase 2 study (NCT00866073), indicating that the gene signature is specific to the combination. The ONV-DAC signature was also applied to the BeatAML cohort (n=399) and identified 241 predicted responders. Mutation analysis of the BeatAML cohort revealed a positive association between predicted ONV-DAC response and mutations in splicing factors (SF) such as SRSF2, suggesting that SF mutations may serve as a response biomarker for the ONV-DAC combination. Indeed, genomic analysis of baseline BM samples from patients evaluable for efficacy in our Phase 1b/2 (n=46), showed a significant increase in clinical responses in patients with SRSF2 and SF3B1 mutations versus patients with other alterations (Figure 1 and Table 1). In addition, biomarkers reported to be positively correlated with DAC responses (TP53 and TET2 mutations, high fetal hemoglobin expression) were not associated with ONV-DAC clinical responses (Figure 1).
Conclusion: We identified a gene expression signature associated with clinical response to the ONV-DAC combination, that did not predict response to DAC single agent. The ONV-DAC predictive signature was found to be correlated with SF mutations in the BeatAML cohort. In our cohort, we confirmed the association between clinical response to ONV-DAC and SF mutations. These analyses support further investigation of the ONV-DAC combination in R/R AML patients with SF mutations and the potential use of ONV-DAC for other hematological diseases with high prevalence of SF mutations such as myelodysplastic syndrome and chronic myelomonocytic leukemia.
Zeidan: Janssen: Consultancy; Astellas: Consultancy; Astex: Research Funding; Genentech: Consultancy; Daiichi Sankyo: Consultancy; Aprea: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; Gilead: Consultancy, Other: Clinical Trial Committees; Epizyme: Consultancy; BeyondSpring: Consultancy; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Pfizer: Other: Travel support, Research Funding; BioCryst: Other: Clinical Trial Committees; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding; Geron: Other: Clinical Trial Committees; Kura: Consultancy, Other: Clinical Trial Committees; Ionis: Consultancy; Incyte: Consultancy, Research Funding; AstraZeneca: Consultancy; Jasper: Consultancy; Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Jazz: Consultancy; Amgen: Consultancy, Research Funding; Agios: Consultancy; ADC Therapeutics: Research Funding; Acceleron: Consultancy, Research Funding; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding. Ridinger: Cardiff Oncology: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Croucher: Cardiff Oncology: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Samuëlsz: Cardiff Oncology: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Erlander: Cardiff Oncology: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Ruffner: Cardiff Oncology: Current Employment, Current equity holder in publicly-traded company; ALX Oncology: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; Gilead: Current equity holder in publicly-traded company; BMS: Current equity holder in publicly-traded company; Anthem INC: Current equity holder in publicly-traded company; Cardinal Health: Current equity holder in publicly-traded company; Change Healthcare: Current equity holder in publicly-traded company; CVS Health Corp: Current equity holder in publicly-traded company; Merck: Current equity holder in publicly-traded company; Organon: Current equity holder in publicly-traded company. Wang: GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Mana Therapeutics: Consultancy, Honoraria; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory board; DAVA Oncology: Consultancy, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Advisory Board; Rafael Pharmaceuticals: Other: Data safety monitoring committee; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal